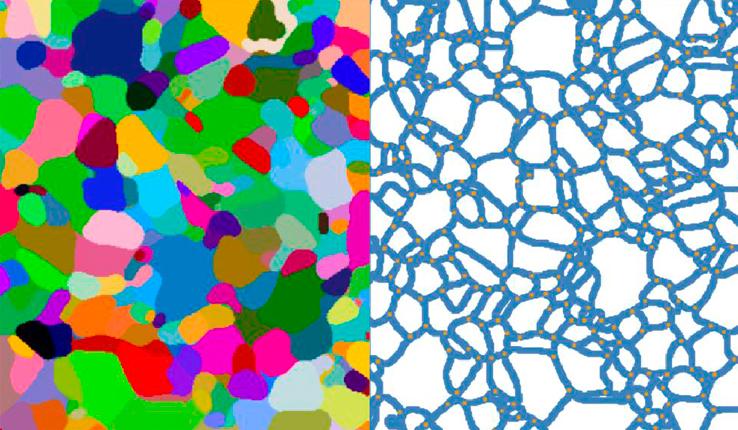
Catalysts, that make chemical reactions occur faster and more efficiently, are used in the production of most of the chemicals people use daily, such as gasoline, fertilizers, and even margarine. The problem? Many of these catalytic processes require a tremendous amount of energy, often in the form of heat, and generate harmful byproducts. Furthermore, most of these products are still produced from fossil fuels.
Researchers from Lehigh University in the U.S. and Cardiff University in the U.K. have pioneered a bimetallic catalysis technique using gold and palladium nanoparticles on a conductive support that could lead to new design pathways for greener production of renewable bio-derived fuels and chemicals.
The results of this collaboration are described in a paper, “Au–Pd separation enhances bimetallic catalysis of alcohol oxidation” published recently in the journal Nature. The authors include, from Lehigh: Steven McIntosh, Department of Chemical and Biomolecular Engineering, and Christopher Kiely, Department of Materials Science and Engineering; and, from Cardiff: Graham J. Hutchings, Max Planck-Cardiff Centre on the Fundamentals of Heterogeneous Catalysis FUNCAT.
Gold and palladium are among the most promising elements used in catalysts for upgrading bio-derive molecules to useful chemicals. The trick is that each element is active for different reaction steps in the upgrading process, explains McIntosh. In this case, gold is active for alcohol dehydrogenation but not active for oxygen reduction. Palladium is the opposite. These elements are often combined into single particles to create a 'compromise' catalyst, he says.
McIntosh and his colleagues discovered an unexpected enhancement in bimetallic catalyst activity using these two elements that is attributable to particles of each element ”talking” to each other via electron transfer through the conducting support. They demonstrate how, by separating the gold and palladium components in bimetallic carbon-supported catalysts, they achieved almost double the reaction rate compared to that achieved with the corresponding alloy catalyst.
“There was a dramatic increase in rate―above the rate of each one separately―when the two different types of particles were placed together on a conducting support enabling the electron transfer between them,” says McIntosh. “Unraveling why this happens took a huge amount of very thorough research, checking and rechecking hypotheses until we reached the conclusions presented in this paper.”
The collaboration brought together electrocatalysis and thermal catalysis to understand and design novel catalyst systems.
“This hybrid approach meant that we could not only understand where the particles were on the support materials, but also bring electrochemical techniques to bear to demonstrate the mechanism and driving forces for this process,” says McIntosh.
This discovery could provide a pathway to design and understand similar bimetallic catalyst systems for a wide range of reactions, says McIntosh, adding: “It has the potential to change the way experts think about the design and creation of these catalysts for many applications―in this case, for the green production of bio-derived chemicals and fuels.”