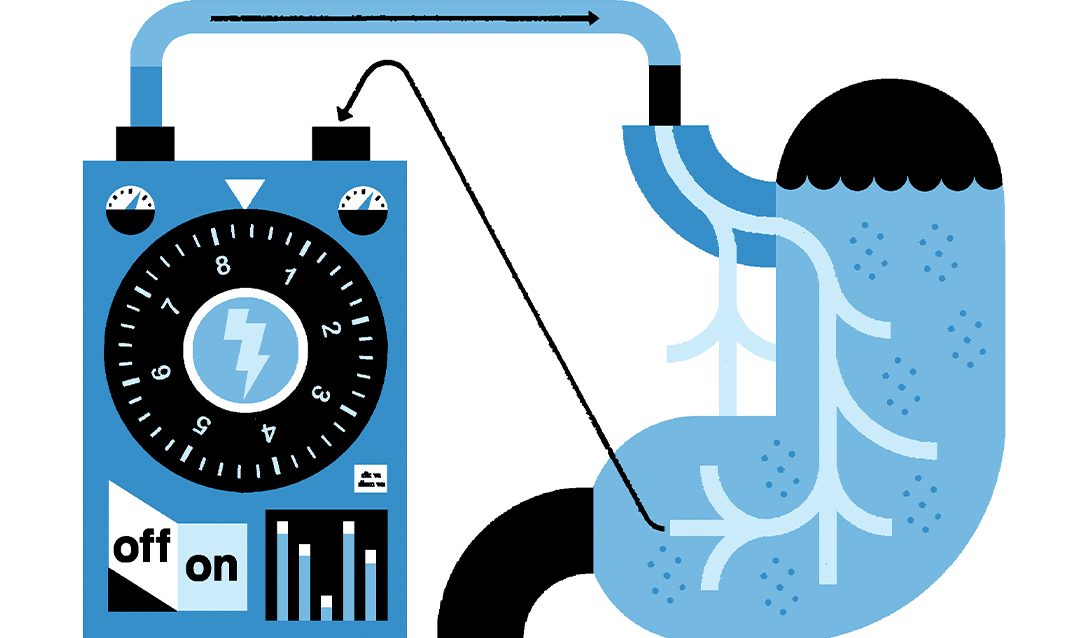
“...Now the grand challenge is how do you design these electrical signals so that they do what you want them to do?” he says. “Because if you send the wrong neural signal, it could create a worse symptom. Just like with drugs … a drug can have a good effect or a bad effect. How do you design a drug that is going to give you the right beneficial effect? So that's the whole purpose of this [research] program: understanding what stimulation does to an organ, developing predictive mathematical models of this behavior and changing the stimulation in such a fashion that it actually [treats the organ’s disorder].”
Kothare and Babak Mahmoudi, a machine learning scholar from Emory University, are co-principal investigators on the project. The co-investigators are Charles Horn, a neuroscientist from the University of Pittsburgh; Raj Vadigepalli, a professor of pathology, cell biology and anatomy from Thomas Jefferson University; and Gautam Kumar ’08G ’13Ph.D., an assistant professor of chemical and materials engineering at San Jose State University. Mark Arnold, a retired Lehigh computer science faculty member, helped develop novel approaches to software and cloud-based simulations for the project. Several students and postdocs from across the five institutions are also collaborating on the work.
The group is using a two-pronged approach. Horn is collecting experimental data by implanting electrodes in the gastrointestinal system of ferrets to stimulate the gastric nervous systems. Team members are also analyzing existing data, such as cardiac rhythm data collected from dogs by the University of California, Los Angeles, which is where the modeling, machine learning, and cloud computing pieces come in.
“What we are trying to do is to develop mathematical models of the behavior of the organ in response to different stimuli, and then use these mathematical models to develop the best or 'optimal' signal that could eliminate the disorder,” Kothare explains. “So that requires us to have an understanding of the underlying biophysics. How do the muscles contract? … How does the contraction of the muscle move the liquid inside the stomach? What prevents the liquid from flowing out of the stomach continuously? … So, these are the kinds of quantitative, almost engineering-like questions we are asking to understand in the form of equations and models—how we can predict the behavior of organs in response to stimulation. When we don’t fully understand the underlying biophysics, we employ data-driven machine learning models to capture the observed behaviors.”
The work involves creating cloud-based solutions for the organ models using machine learning and model-based control algorithms, and the testing of these tools through mathematical simulations carried out by linking computers in different geographical locations through cloud- and web-based computing. The cloud-based and distributed simulations allow collaborators developing organ models to link remotely with control and optimization experts developing therapies to test new algorithms and eventually also link remotely with stimulation and measurement device manufacturers. Kothare says Arnold centralized the team’s working environment by connecting the computers at each of the researchers’ institutions through the cloud so they could streamline and leverage their data analysis from the various algorithms and models, an important milestone that vitalized their collaborative efforts. The modularity of the organ models and the neuro stimulation therapy algorithms will allow these models to be used by other researchers in the future, particularly through open access through cloud simulations, GitHub and other repositories.
“...One of the things we realize is we simply cannot solve everything,” says Kothare. “The human body is so complex, we have to depend on pieces of discoveries and pieces of models developed before by other groups, combine them and see if we have answers to developing equations for the different components of the organ.”
Closing the Loop
A distinctive aspect of the project’s approach, and one of Kothare’s key contributions, was designing, analyzing, prototyping, deploying and executing feedback control architectures for the closed-loop—rather than open-loop—stimulation of the peripheral nervous system. In open-loop systems, the same signal is injected, independent of the response. In closed-loop systems, the input signal is dependent on the measured output or response. Kothare says for this reason, open-loop approaches are not as effective because they are not capable of adjusting to meet the body’s needs. If less medication or stimulation is needed to achieve a stabilized heart rhythm, for instance, a closed-loop system would adapt.
“So the increasing trend now is even for the pacemaker, there is a closed-loop or responsive pacemaker,” Kothare explains. “So in a closed-loop system, the pacemaker not only generates a signal that goes to the heart but also gets a reading about the heart rate. So it says, okay, you know, the heart rate has gone very high. I don't need to [add] so much stimulation, I can reduce the stimulation. … So [when] information is used to change the stimulus signal, it is called closed-loop. … And so [Mahmoudi and I] are pushing that [closed-loop] paradigm as an important paradigm for developing these therapies.”
Arnold, Kumar, Kothare, Mahmoudi and additional collaborators published the first major paper on their research in March 2022: “CONTROL-CORE: A Framework for Simulation and Design of Closed-Loop Peripheral Neuromodulation Control Systems” in Volume 10 of IEEE Access.
Similarly, a paper that employs a deep learning model to describe cardiovascular response and uses the model for designing a predictive closed-loop cardiac control algorithm was reported in another article in June 2022: “Data Driven Control of Vagus Nerve Stimulation for the Cardiovascular System: An in Silico Computational Study” in Frontiers of Physiology.
Kothare, a control theory engineer who previously served as visiting professor of biomedical engineering at Johns Hopkins School of Medicine, says he found in the area of biomedical engineering abundant opportunities to apply his control systems background. The human body relies on feedback systems to maintain homeostasis, he explains. Our glucose, creatinine, salt, and sugar levels, for example, all fall within a specific range by design. Machine learning has enabled an explosion of technology for artificial intelligence-based modeling, adding to the low-hanging fruit for research.
“All of a sudden, all these models that we found difficult to develop—now they can be developed automatically with algorithms even without understanding the entire behavior,” Kothare says. “So all these factors brought me into this area, and I'm learning every day.”
Mayuresh Kothare’s interdisciplinary research interests span the problems of constrained and optimal predictive control theory, closed-loop robustness analysis, modeling and machine learning control of chemical, biomedical and neuro engineered systems. He previously served as the Chair of the Department of Chemical & Biomolecular Engineering from 2012-2021. He received his Ph.D. from the California Institute of Technology.