Secret to Green PVC Catalyst Revealed
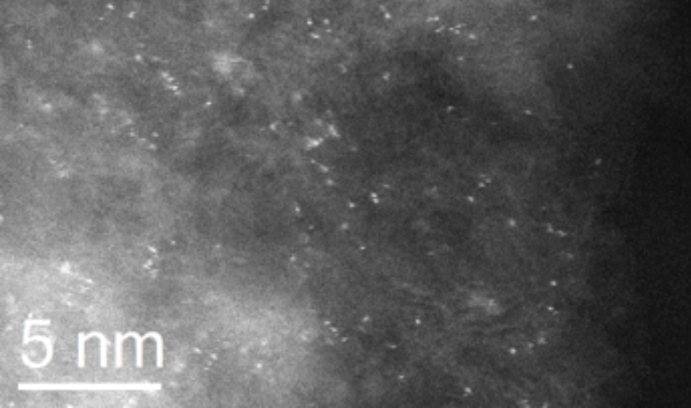
An image obtained with scanning transmission electron microscopy and high-angle annular dark-field imaging shows isolated species of gold on a carbon support. The Cardiff-Lehigh team found that atomically dispersed atoms of gold achieve acetylene conversion most effectively. (Image courtesy of Christopher Kiely)
An international group of scientists has unlocked the secret of a gold-based catalyst that is responsible for a new, environmentally friendly method of producing the vinyl chloride monomer (VCM) that is used to manufacture polyvinyl chloride (PVC).
Using synchrotron-based spectroscopy techniques and advanced electron microscopy, the researchers have determined that isolated gold ions most effectively convert acetylene, a gas derived from coal, to the VCM molecules that can be subsequently linked to form PVC. PVC is the world’s third-most widely used plastic.
Their discovery comes amid efforts to replace the conventional method of acetylene conversion, which uses a volatile and potentially toxic mercury-containing catalyst, with a more stable, non-polluting method that employs a carbon-supported gold catalyst.
The researchers, who are from the United Kingdom and the United States, reported their findings today (March 30) in Science magazine, the world’s premier science journal, in an article titled “Identification of single site gold catalysis in acetylene hydrochlorination.”
The article’s lead author is Grazia Malta of the Cardiff Catalysis Institute at Cardiff University in the UK who was supervised by Graham J. Hutchings, the Institute’s director. The Lehigh University participants in the research were Christopher J. Kiely, professor of materials science and chemical engineering and Li Lu, a Ph.D. candidate in materials science. Kiely is also a co-director of the Cardiff Catalysis Institute.
The group examined the catalysts before and after use in Lehigh’s aberration-corrected JEOL JEM-ARM200CF scanning transmission electron microscope (STEM), which is one of the most powerful instruments of its kind and allows imaging and chemical analysis of materials at the atomic level.
The group also performed extended x-ray absorption fine structure (EXAFS) and x-ray absorption near edge structure (XANES) experiments using the Diamond Synchrotron Facility in the UK to study the catalyst under working reaction conditions.
“These experiments helped us determine that atomically dispersed gold—where the atoms are separated on the carbon support and are not touching—is the ideal form of catalytic species for this reaction,” says Kiely.
“They also showed us that you need the gold atoms to be ionized—that is, missing some of their electrons—for the conversion to occur.”
Led by C. Richard A. Catlow of the Cardiff Catalysis Institute and University College London, the group also theoretically modeled the reaction using isolated gold ions and confirmed the experimental results.
“Scientists have known that you can use atomically dispersed gold in homogeneous catalyzed reactions perfomed in solution,” says Kiely. “Here, we have managed to anchor atomically dispersed gold on a solid support and achieve a similar effect.”
Kiely and Hutchings, who have collaborated for several decades, reported in an article in Nature Communications last year that for another reaction, namely the low temperature oxidation of carbon monoxide to carbon dioxide, a different gold entity—ultra-small clusters consisting of a few gold atoms—were the most active species.
The results of both these projects will help Kiely and Hutchings design and optimize gold-based catalyst systems for use in other important reactions, such as the water-gas-shift reaction, which generates hydrogen.
A 30-year campaign to replace mercury
PVC has become an indispensable part of modern life. Its applications include construction pipes, credit cards, window and door frames, plumbing equipment, and electrical cable insulation.
In addition to acetylene hydrochlorination, the VCM molecule precursor to PVC can be made from ethylene, a by-product of petroleum refining that can also be isolated from natural gas. But acetylene hydrochlorination remains the predominant path to PVC production in some countries that have abundant coal reserves.
To convert coal-derived acetylene into the VCM precursor, says Kiely, chemical engineers for the last half-century have reacted it with hydrochloric acid (HCl) in the presence of a mercuric chloride catalyst. But the catalyst is volatile at reaction temperatures, allowing the toxic mercury to evaporate, escape into the environment and pollute farmland and water bodies.
In the early 1980s, Hutchings showed that a more benign, carbon-supported gold catalyst could be used to convert acetylene to VCM. His discovery attracted some attention at the time, but was not commercially exploited as the catalyst required relatively large amounts of expensive gold and was not very stable.
In 2007, Johnson Matthey, a global speciality chemicals company based in the UK, became interested in Hutchings’ results and began working to make a stable gold-on-carbon catalyst using less gold. The company developed a catalyst named Pricat MFC, which has now gone into commercial use in a large Chinese PVC plant. China, the world’s largest producer and consumer of PVC, still relies on coal to produce the VCM product.
Meanwhile, the 2013 Minamata Convention on Mercury, which has been signed by nearly 140 nations, bans the construction of new VCM plants utilizing mercuric chloride after 2017 and requires all VCM plants to be mercury-free by 2022.
Hutchings’ early work, the commercialization efforts by Johnson Matthey and the most recent discovery of the atomic-scale workings of the carbon-supported gold catalyst, says Kiely, give cause for hope that the goals of the Minamata Convention can be attained.
They also represent a nearly unprecedented achievement in the field of catalysis.
“Scientists are always tweaking and optimizing catalyst formulations,” says Kiely. “But, this is the first time in 50 years that I can recall where we have replaced an industry-standard catalyst used in a major reaction with an entirely different catalyst system.”
Story by Kurt Pfitzer
Posted on:




