An elaborate, and vital, choreography
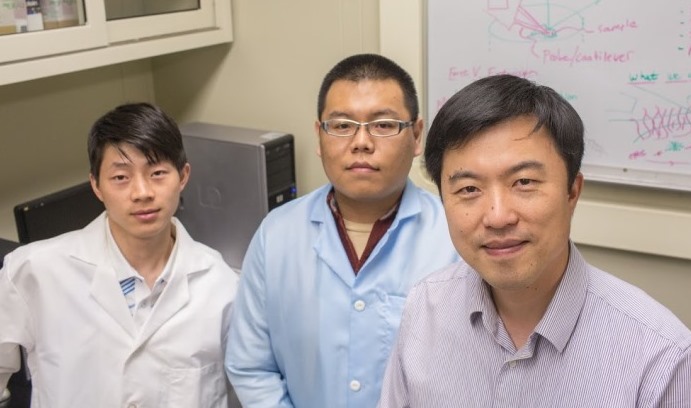
Wei Zhang, Yan Xu and Frank Zhang (l-r) recently published an article in the journal Blood that sheds light on the mechanical forces that activate platelets and instruct them to bind to injured blood vessels.
From shaving nicks to paper cuts to lacerations, every rupture of a blood vessel triggers a chain reaction of sensing, signaling, anchoring and clustering as the body’s cells and proteins mobilize to form a blood clot.
The actors in this drama, says Frank Zhang, achieve an elaborate choreography enabling platelets—the tiny cell fragments that respond first to a wound site—to form a plug and then a thrombus that seals the wound.
Scientists know what happens at the molecular level to bring about blood clotting, but they have been unable to explain how platelets sense the mechanical force that activates them and instructs them to adhere to injured blood vessels.
Zhang, an assistant professor of mechanical engineering and mechanics, and his colleagues recently shed light on this phenomenon by identifying a critical interaction involving platelets, flowing blood and the protein, von Willebrand factor (VWF), which facilitates clotting and prevents unchecked bleeding.
Using a laser technique called optical tweezers, the group has isolated and immobilized individual platelet and VWF molecules, subjected them to very slight mechanical forces and observed their interaction. They have discovered a “hitherto unidentified mechanosensitive domain” (MSD) in a protein molecule on the surface of the platelets.
The discovery, says Zhang, could have positive implications for the treatment of von Willebrand diseases, a group of genetic coagulation disorders caused by a deficiency or mutation of a person’s VWF molecule.
“Our hope is that this will help lead to the development of a drug that will treat or prevent disease,” he says, “either by protecting MSD or by preventing the unnecessary activation of platelets.”
Zhang and his colleagues, who have received funding from the National Institutes of Health, recently reported their findings in Blood, the journal of the American Society of Hematology. Their article was titled “Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex.”
How platelets sense shear stress
The process by which blood coagulates takes place at multiple levels, says Zhang, who is also affiliated with Lehigh’s bioengineering program.
Platelets travel to the site of a wound, but the fast flow of blood makes it difficult for them to bind to the collagen beneath the ruptured layer of endothelial cells. So von Willebrand factor (VWF), a fiber-like protein molecule named for a 20th-century Finnish pediatrician, anchors itself to collagen and then pulls platelets from the blood.
VWF, says Zhang, has 12 domains, including three A domains—A1, A2 and A3—that are essential to platelet adhesion. While A3 binds to collagen, A1 binds to GPIb-IX, a protein molecule on the platelet surface. Scientists have known for 20 years that GPIb-IX acts as a platelet’s mechanosensor, sensing the shear, or sideways, stress of flowing blood. Scientists have also learned that the binding of A1 and GPIb-IX causes a platelet to elongate from a sphere into a flatter particle, to extrude filopedia that stick to the collagen, and to stack on top of other platelets to form a plug.
Until now, however, no one has explained how a platelet senses the shear stress and how it interprets and acts on this information, Zhang’s group wrote in their article.
“How this [GPIb-IX] receptor complex senses shear stress and converts this mechanical information into a protein-mediated signal that can be recognized and propagated [by the platelets] has remained elusive,” the group wrote.
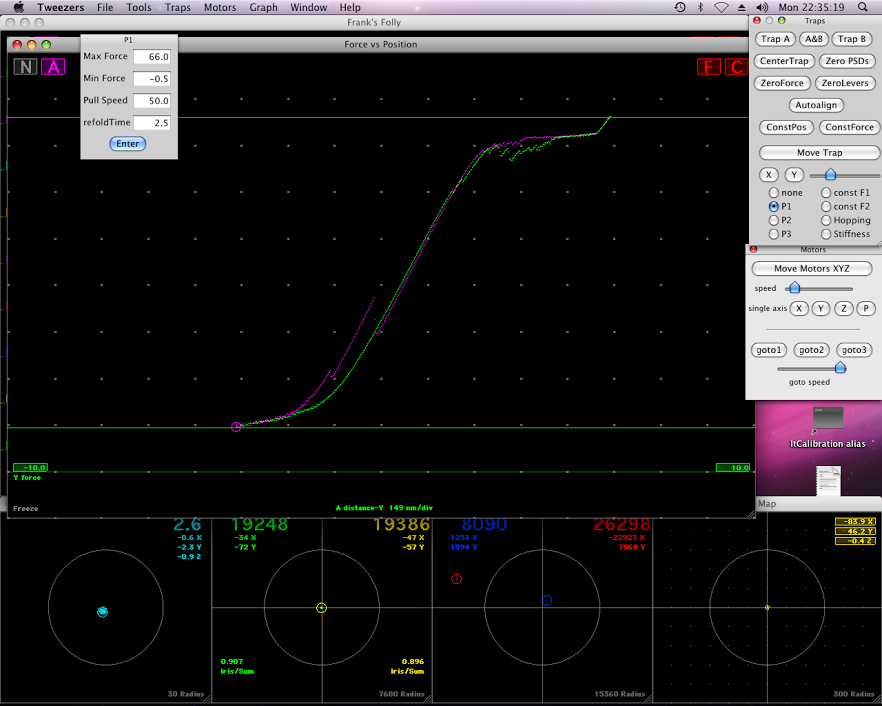
Zhang and his students explore this phenomenon using optical tweezers. By focusing a laser with a large light spot into a tiny area, they concentrate its intensity and enable it to pin and immobilize a bead, or sphere, measuring 2 microns in diameter.
“We immobilize VWF on one sphere and GPIb-IX on another,” says Zhang. “We bring the two spheres together and watch the two molecules interact.
“We use an optical fiber with a nanopositioner to move the bead. We move the trap slowly to the left and the force slowly increases. We can sense the position of the sphere and measure the force. The distance that the sphere moves from the trap in the center is proportional to the force that we apply.
“We do this with a one-nanometer precision; we apply tens of picoNewtons of force.”
In this manner, says Zhang, his group has determined that the GPIb-IX extends in an “alpha chain” from the platelet membrane and that the end of this chain binds to VWF-A1. The force of the flowing blood tugs on this chain and causes a portion of the chain—the “hitherto unknown” MSD—to unfold, stretch away from the VWF and disrupt the structure. As it unfolds, the MSD sends a signal to activate the platelet.
“The end of the MSD stretches away from the VWF as blood flows past,” says Zhang, “very much like a ship’s anchor, which stretches or pulls away from the ocean floor in response to the force of the ocean waves.
“During this stretching, the MSD, which is a protein with a 3D amino acid structure, unfolds until the structure is disrupted.”
Using the optical tweezers, Zhang’s group was able to identify the threshold force necessary to disrupt the MSD. They attached one sphere to the platelet and one to the VWF and pulled the two spheres apart very slowly, gradually increasing the force until the elastic structure (the MSD) suddenly popped apart into its unfolded phase.
“In our optical tweezer experiment we have observed the binding and unbinding of VWF-A1 and full-length GPIb-IX at the single-molecule level,” the group wrote in its article. The researchers added that they were the first to take a “single-molecule force measurement of the full-length GPIb-IX complex.”
Its results, the group wrote, “suggest that VWF-mediated pulling under fluid shear induces unfolding of the mechanosensitive domain in GPIb-IX, which may possibly contribute to platelet mechanosensing and/or shear resistance of VWF-platelet interaction.”
Zhang is seeking additional NIH funding to study platelet activation and VWF diseases in greater detail.
The current project is a collaboration involving researchers from Lehigh, the Aflac Cancer and Blood Disorders Center of the Emory University School of Medicine, the University of Texas Health Science Center at Houston, and the Puget Sound Blood Center in Seattle.
The lead author of the Blood article is Wei Zhang, a Ph.D. candidate at Lehigh. The other Lehigh authors are Frank Zhang; Yan Xu, a Ph.D. candidate; and Yizhen Wang, a former postdoctoral researcher who is now an associate professor of physics at the University of Hainan.
The Emory authors are Renhao Li, an associate professor in the university’s School of Medicine who has studied the molecular structure of GPIb-IX for 12 years; Wei Deng and Xin Liang, postdoctoral research fellows; and Liang Zhou, a senior research scientist. The remaining authors are Wenjun Yang of the University of Texas Health Science Center and John D. Kulman of the Puget Sound Blood Center.
Story by Kurt Pfitzer
Photos by Christa Neu
Posted on: