A Potential New Weapon Against Superbugs
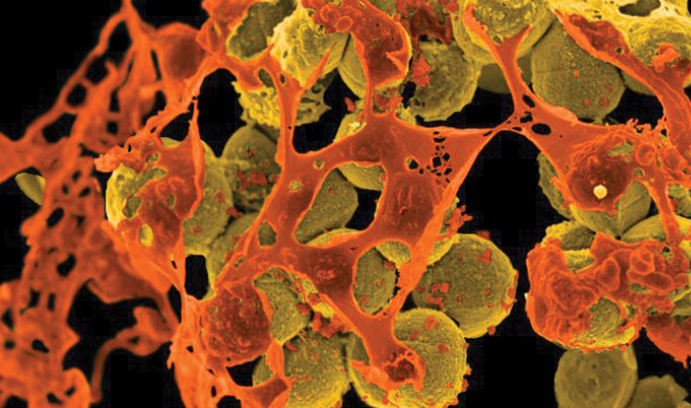
This digitally-colorized image, taken with a scanning electron microscope, shows mustard-colored, spherical methicillin-resistant Staphylococcus aureus (MRSA) bacteria surrounded by orange cellular debris. MRSA, which can cause skin and respiratory infections and food poisoning, is one of 12 bacteria that are growing resistant to the drugs available to treat them, according to the World Health Organization. (Courtesy of the National Institute of Allergy and Infectious Diseases (NIAID))
Modern medicine, says Steven L. Regen, professor of chemistry, is losing the war against “superbugs” that have built up a resistance to antibiotics.
Public health experts agree. Last week, the World Health Organization listed 12 drug-resistant bacteria which pose the greatest threat to human health and which could soon be untreatable with existing medicines. These include bacteria that cause salmonella, sepsis and pneumonia.
Last year, a report commissioned by the British government warned that drug resistance, which is caused by the overuse or misuse of antibiotics in humans and animals, could become a greater threat than cancer.
Already, according to the report, as many as 700,000 people around the world die each year from drug-resistant infections. That includes 25,000 Europeans, according to the European Food Safety Authority and European Center for Disease Prevention and Control, and 23,000 Americans, according to the United States Centers for Disease Control and Prevention.
One potential solution to the problem of drug resistance, says Regen, is to improve the drugs that kill microbes by disrupting their cell membranes. Bacteria should be less likely to develop resistance toward these drugs, Regen and his research group wrote recently in an article in the journal Bioconjugate Chemistry, than toward drugs that must be internalized to exert their toxic effects.
The article, titled “Simple Strategy for Taming Membrane-Disrupting Antibiotics,” focused on Amphotericin B (AmB), a drug which is highly effective in treating fungal diseases—and which has encountered little resistance from pathogens.
The article’s lead author is Yuming Yu, a former postdoctoral researcher in Regen’s group. Other authors are Regen; Mary J. Sabulski, a Ph.D. candidate at Lehigh; Marcos Pires, assistant professor of chemistry at Lehigh; and Wiley A. Schell and John R. Perfect of the Duke University Medical School.
AmB kills fungal cells by rupturing their outer membranes, says Regen. It is the strongest remedy for several systemic fungal infections, including cryptococcal meningitis and some types of valley fever and yeast infections, but it can cause severe side effects, including fever, chills and headache. And despite its wide use as an anti-fungal agent for more than 50 years, Regen’s group wrote in Bioconjugate Chemistry, “resistance to this antibiotic has proven to be extremely rare.”
“AmB has been the gold standard for treating fungal infections in the last 50 years,” says Regen, “but in some cases it ruptures not only the membranes of fungal cells but those of human cells as well. For this reason, it’s sometimes called ‘Ampho Terrible’ by clinicians.”
AmB can exist as single molecules, or monomers, or as aggregates of molecules, says Regen. Monomers typically puncture the cell membrane, causing a leak and the eventual death of the cell. They selectively target fungal cells but not mammalian cells. Aggregates, by contrast, penetrate to the membrane interior, causing the cell to “blow apart.” Aggregates are less selective, attacking fungal and mammalian cells alike.
Regen and his research team have designed a strategy that reduces the rupturing power of AmB aggregates while allowing AmB monomers to kill fungal cells selectively. To “tame” the aggregates, they link AmB covalently with cholic acid, an insoluble bile acid which has been reported to lie flat on the surface of lipid membranes.
This alignment along membrane surfaces, the researchers reported in Bioconjugate Chemistry, “suggested to us that such molecules could be used as ‘floats’ that would help prevent deep penetration of aggregates of membrane-disrupting agents, thereby reducing their rupturing power.”
Regen and his group tested their hypothesis on the red blood cells of sheep and found that when they treated the cells with low concentrations of AmB by itself, the cells released all of their hemoglobin, a sign of catastrophic rupture. When treated with the conjugate of AmB and cholic acid, however, the cells retained their hemoglobin—even at much higher AmB concentrations than would be required to treat fungal infections.
“The results were pretty dramatic,” says Regen. “The red blood cells that were treated with the aggregates of tamed AmB did not rupture at all. However, the cells that were treated with the AmB alone did rupture.”
Regen’s current study was funded by a grant from the National Institutes of Health. It is a continuation of work he began in the 1990s which culminated with the publication of two articles in the Journal of the American Chemical Society.
In the first article, which was published in 1993 and titled “Control over vesicle rupture and leakage by membrane packing and by the aggregation state of an attacking surfactant,” Regen and his collaborator, Yiping Liu, revealed a fundamental difference between the membrane-disrupting actions of monomers and aggregates of Triton X-100, a commonly used surfactant laboratory detergent.
In the second article, published in 1995 and titled “Micelle/monomer control over the membrane-disrupting properties of an amphiphilic antibiotic,” Regen’s group showed that AmB could be made more selective in killing fungal cells by chemically modifying it to reduce its tendency to form aggregates.
“We did a lot of work in this area in the 1990s,” says Regen, “and then went in other directions. We’re revisiting it now in part because we want to see if it’s possible to extrapolate our results with fungal cells to anti-bacterial agents that disrupt cell membranes.”
Lehigh has filed a patent application for the AmB-cholic acid and related conjugates and is hoping to form a partnership with a drug-development company to test the conjugate in animals.
“We need to have a suitable formulation of our compound made to find out how its biological activity compares with that of other formulations of AmB that are currently in use,” says Regen.
Story by Kurt Pfitzer
Posted on:




