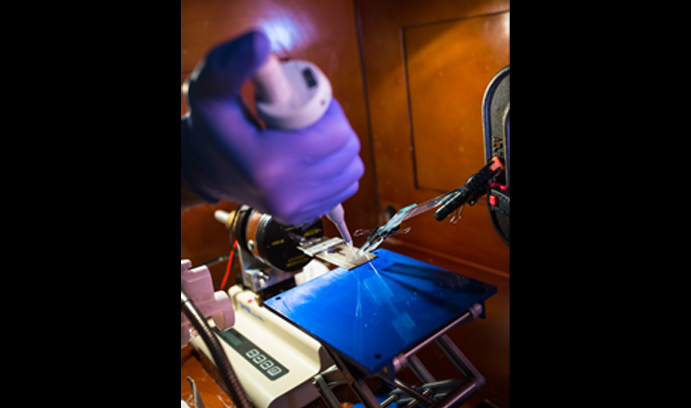
A Useful State of Suspension

A rainbow of colors is generated by silica particles coating a silicon wafer in Lehigh's Laboratory for Particle Mixing and Self-Organization, led by associate professor James Gilchrist.
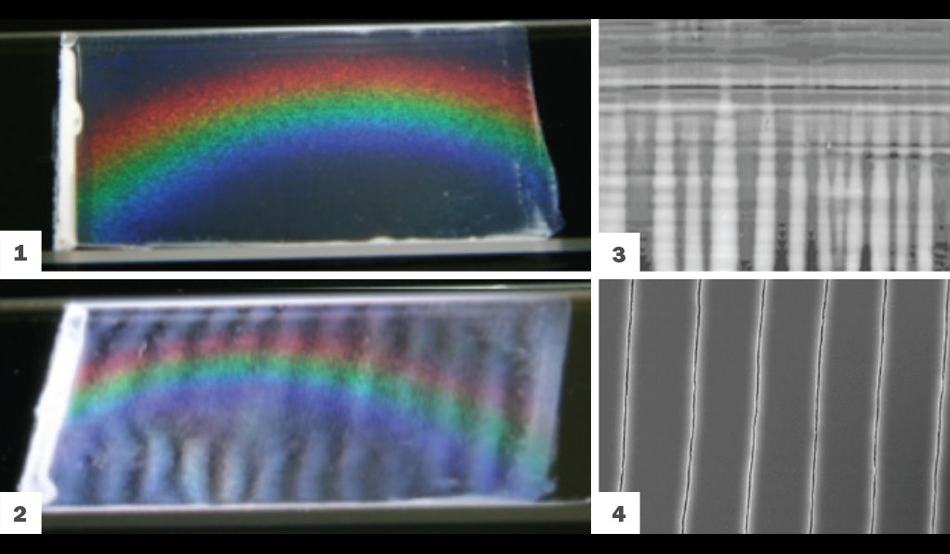
Image 1 shows ideal coating. Image 2, 3 and 4 show the stripes, streaks, and cracks that form when the process goes amiss.

James Gilchrist, Class of 1961 Associate Professor of Chemical Engineering.

(from left to right) Graduate students Tanyakorn Muangnapoh, Tharanga Perera, Midhun Joy, and Kedar Joshi of the Laboratory for Particle Mixing and Self-Organization.
“This is as much art as it is science,” says James Gilchrist, the Class of 1961 Associate Professor of Chemical Engineering. To the uninitiated, it might appear no small part magic as well.
Gilchrist focuses on a process—placing infinitesimally thin films of material onto substrates—that is deceptively simple but loaded with complexity and possibility.
Using a combination of high-end instruments and improvisational tools, he and his students explore the chaotic intricacies of particle behaviors, and a host of potential applications that these behaviors make possible.
In the Laboratory for Particle Mixing and Self-Organization, Gilchrist works with a team of Ph.D. candidates and a senior polymers researcher. Since the field is wide open the work demands creativity as well as discipline.
“There is much that we don’t know yet about the structure and dynamics of the systems of suspensions and granular flow,” Gilchrist says. “We can visualize a lot, but we can’t visualize everything, so we have to imagine what’s going on behind the scenes and what physics are impacting the interactions. That’s where the creativity comes in.
“On the other hand, if we aren’t disciplined in our experiments, our results won’t let us see these physics in action.”
The films that Gilchrist’s group creates typically begin as fluid suspensions of minute particles that are often chosen for unique qualities that will yield a specific product or data. The suspensions are microscopically deposited or applied via a secondary carrier fluid. The suspensions are microscopically deposited or applied via a secondary carrier fluid. A considerable number of outcomes is made possible by manipulating the materials and the process, and the products emanating from the lab are highly sought after.
The variables in play in the manufacturing process are myriad. The ambient temperature, evaporation rate, surface tension and viscosity of the suspension fluid impact the final particle structure in the films. In addition, the speed and angle of the application plate, the qualities of the substrate, and the particle size can all be finely tuned. The goal of these manipulations is to reveal the essential characteristics of the process and the dynamics of complex interactions of suspensions.
What makes much of the group’s work not just viable but valuable is a remarkable self-organization that frequently occurs, or is made to occur, as particles are applied during film deposition. Thanks to microscopic imaging, the researchers can watch a wedge scrape a fluid suspension loaded with particles across a flat surface. The layer of film that is left behind is a single particle thick. The suspended particles sort themselves into perfect arrays. Sometimes, when the array is inconsistent, the particles even correct and snap into place on the grid after a momentary delay once the wedge has moved past.
The seemingly magnetic––and fortuitous––phenomenon has been dubbed the “Cheerios effect.” Just as bits of breakfast cereal floating in a bowl of milk will cling together, particles in a suspension can attract each other. The behavior is a combination of two factors. As particles are trapped in the film, they form dimples in the fluid’s surface and drag neighboring particles toward them. In addition, because of the difference in pressure between fluid and air, the fluid surface can act like a membrane. This membrane resists objects that are pressed into it, causing further pressures that buffet suspended particles as a delicate equilibrium is formed where air and the denser fluid meet.
Particle structures in thin films are often hexagonal in form, though sometimes square (called cubic packing), the latter being less common but having useful properties. “If someone needs that, we can dial it in by changing the experimental conditions,” Gilchrist says.
Capturing light from LEDs…
One application for the particle structures that Gilchrist’s group creates is the light-emitting diode, or LED, which is gaining popularity as a domestic lighting choice because of its superior energy efficiency. Gilchrist was approached by Nelson Tansu, associate professor of electrical and computer engineering, who produces high-end LEDs and wanted to make them brighter. The two researchers hypothesized that a particulate coating on the surface of an LED could improve light extraction within the LED—if well-chosen particles behaved like miniature lenses.
“The inside of an LED is a high-index refraction material, while air is low-index refraction,” says Gilchrist. “Our first idea was to just use a rough surface to refract light, and extract it from the LED that way. We created a film––basically a microlens array–– where the particles were the same gauge as the wavelengths of the light being emitted, which is where the efficiency comes in.”
The researchers aimed for a 5- to 10-percent increase in the amount of light emitted from the LED. In their first trial, the LEDs produced twice the amount of light.
Gilchrist now leads a group that is attempting to perfect the film. “The challenges are to make it more uniform and more efficient and to scale it up,” he says. The group, which includes Mark Snyder, assistant professor of chemical engineering, has received a $1.1 million grant from NSF.
Eric Daniels, a research scientist, is working with Gilchrist on the NSF project, among others. He anchored the lab during Gilchrist’s recent sabbatical at the California Institute of Technology, and his prior experience at Lehigh’s Emulsion Polymers Institute has proved helpful, especially as EPI has been a major resource for many of the projects at the lab.
“We frequently use parts synthesized by EPI,” says Gilchrist. “They are a crucial partner. The particles we use have to be uniform and manufactured to a critical tolerance. We could not do these projects without them.”
One of Daniels’ main tasks has been preparing a recently acquired piece of hardware, an Automated Langmuir-Blodgett, for operation. Though it looks no more sophisticated than a stainless steel printing press, it has the potential to deposit a microscopically fine layer of particles using a wet-on-wet technique at high speed. With a mechanism that resembles a conveyer belt, the machine lays a suspension evenly on a fluid-laden substrate passing beneath it.
…and pathogens from the bloodstream
The potential applications for thin films are so rich that, rather than having to seek collaborators, opportunities seem to come looking for Gilchrist and his crew. Alexander Weldon, who will complete his Ph.D. in May, is preparing a microfilm for use on a new device being developed by Xuanhong Cheng, assistant professor of materials science and engineering and bioengineering, to enable the capture of blood-borne pathogens.
“We’ll put down layers of particles in their device, and functionalize them using antibodies or something that would bind to, say, a cancer cell,” says Weldon. “The particles will grab pathogens and trigger an irreversible reaction. It could be used as a diagnostic or detection tool. We have to discover how to optimize this through the right particle size and pore size. It’s a very fulfilling collaboration.” Weldon was lead author on a paper in Applied Materials and Interfaces that showed how to employ two particles of different sizes to create a film with pores that could be fine-tuned to filter such targeted pathogens out of blood or other suspensions.
The potential for the work coming out of Gilchrist’s lab is versatile, and it has led to rewarding collaborations with other Lehigh researchers in a wide range of fields. And while more efficient LEDs and solar cells, pathogen detectors, better batteries and higher-resolution HD displays are just some of the beguiling applications of thin films, that’s not how Gilchrist interprets his mandate. “We’re here to do fundamental science,” he says. “We are not going to be the people that coat things—we want to enable others to coat things better.”
One fundamental problem that the group is exploring is the radical change in viscosity and particle behavior that a suspension can undergo when it is sheared. Rubbing a suspension between thumb and forefinger is an example of shearing; as the internal structure changes, it changes the viscosity.
Tharanga Perera, a Ph.D. candidate, is attempting to reconcile empirical results with theoretical calculations on the shearing of suspensions. The calculations have been unresolved for almost 20 years. One challenge is to locate and track individual particles in a suspension. To test and verify the existing numerical calculations, Perera uses sophisticated confocal microscopy that can capture a 3-D nanoscale image at high speed. This allows him to experimentally determine particle location and check the results against computer simulations. To generate more robust data, he makes changes in the experiments that cannot be executed on the computer.
Learning from anomalies…
Along with the successes come equally prized anomalies, which frequently spark innovation. “We can do an experiment where 90 percent of things go the way we anticipate,” says Gilchrist, “but a lot of our most interesting work ends up being driven by the 10 percent we aren’t expecting.”
While working with inorganic, magnesium oxide films, Midhun Joy, a Ph.D. candidate, came across a way of more easily creating cubic-packed structures in the films by changing the substrate properties. His motivation was to avoid the cracks that had been occurring in films placed on less flexible glass carriers. While he was examining a sample done with the polymer substrate, Joy noticed the cubic structure of the particles––a happy, but unanticipated, result.
“We don’t understand exactly why this is happening, so we’re studying the fundamentals of how it occurs,” said Gilchrist. Other engineers have expressed strong interest in the possibilities for this work.
In another recent project, Tanyakorn Muangnapoh, a Ph.D. candidate and Royal Thai scholar, came to Gilchrist with a problem: How could the group deal with unavoidable vibrations from the building or an outside source that affected the manufacturing process as film was being deposited? The group came up with the novel idea of deliberately adding vibrations into the deposition process to determine if they might actually be beneficial.
“It was as if we decided to shake a gumball machine to see if we could get more efficient packing,” Gilchrist says. Muangnapoh tried different parameters and reported positive results.
“He told me the good news, that the vibrations produced these nice monolayer coatings,” says Gilchrist. “The ‘bad’ news was that even when he altered the parameters he still got the monolayer coatings. We weren’t expecting that.”
Muangnapoh, with Weldon and Gilchrist as coauthors, recently described his results in a paper published in Applied Physics Letters. “We still don’t totally know what’s going on,” says Gilchrist. “We understand the heuristics of it, now we need to keep working to find out fundamentally what’s happening.”
…and from failures
Failure is sometimes more than an option; it is actively pursued and analyzed.
“We’ve spent a lot of time in the past year studying how things go wrong as opposed to right,” Gilchrist says. For instance, if something goes wrong during the particle deposition process––if particles of different sizes fall awry, if an impurity is introduced or if a heated substrate causes the film to buckle––the defect can snowball and become irreversible, resulting in stripes or streaking in the film.
Kedar Joshi, a Ph.D. candidate, is seeking to find out if adding surfactants to a suspension will reduce the surface tensions to avoid these instabilities. Gilchrist and his students have created hundreds of failed samples to better understand the minute choreography that takes place in these nanoparticle pileups.
“You can’t predict this stuff,” says Gilchrist. “The LED improvements, in part, came about because a student was dissatisfied with the project he was working on. We‘ve had some major discoveries by dissatisfied students.
“My students are independent. I want them to tell me I’m wrong,” Gilchrist says. “Often, when I have students facing a challenge, I tell them to try a number of things. They often say, ‘I already did.’
“I love that. My attitude is that if you receive your Ph.D. you should be one of the world’s leading experts on your topic. That says all you need to know about the quality of our chemical engineering program at Lehigh.”
Story by Chris Quirk
Photos by Ryan Hulvat
Posted on: