A Naturally Inspired Study of Nanosuspensions

Webb studies the behavior of liquids when they make contact with solids. His specific focus is nanodroplets; he uses computer simulations to explore how atoms at the interface of liquid and solid behave as a droplet spreads.
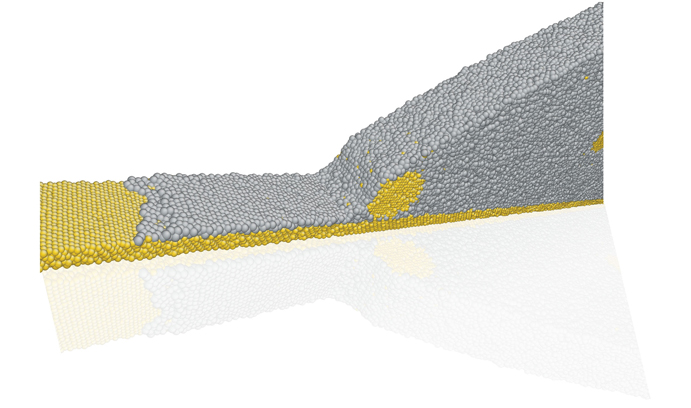
A simulation shows the edge of a nanosuspension droplet that has been pinned. Each sphere represents an atom.
Lotus flowers do it. So does the Namib Desert beetle. Both have a feature that causes water to gather into tiny balls on their surface.
When rainwater hits a lotus plant, the droplets coalesce on top of micrometer-scale bumps on its leaves, then slide off, picking up dirt particles on the way and leaving the leaves clean.
A similar pattern of hydrophilic bumps on a desert beetle allows it to harvest spheres of precious moisture from its back.
These marvels of the natural world fire Edmund Webb’s imagination. An associate professor of mechanical engineering and mechanics, he finds inspiration in the overlap between nature’s innovations and human inventions, such as self-cleaning solar panels that use the lotus effect to remove dirt, and potential exoskeleton technology that could collect water in an arid climate.
Webb studies the behavior of liquids when they make contact with solids. His research reveals the intricacies of the wetting and spreading of droplets that are invisible to the naked eye.
Webb’s specific focus is nanodroplets, including nanosuspensions, or droplets that contain particles. He uses computer simulations to explore how atoms at the interface of liquid and solid behave as a droplet spreads. Particles suspended in a liquid affect droplet spreading behavior; the coffee cup ring is a classic example.
“When you put down a coffee cup that has coffee all over the bottom,” Webb asks, “why does the puddle form a ring-shaped stain instead of a filled-in circle?” Because, he says, all the liquid flows to the edge—where evaporation is the fastest—and carries particles of coffee with it. Toward the end of evaporation, when the liquid would preferentially retract from the edge, it cannot flow back to the middle, because coffee particles pin the liquid edge to the table’s surface.
“All these particles have gathered around the edge, and that’s how the final shape, a ring, is achieved,” Webb says. He points out that, in some systems, particles flow to the edge but are able to flow back so that a different final pattern emerges. “We can use that natural phenomenon as inspiration for what we can engineer.”
Using a Lehigh Faculty Innovation Grant (FIG), Webb and Ph. D. candidate Baiou Shi have explained a phenomenon of droplet spreading using data not previously observed. Prior experiments showed that the liquid front of a spreading droplet could be pinned, or halted, by the presence of suspended particles; however, the forces involved in the process could only be conjectured and not measured. Simulation results from Webb’s group permit the first quantitative extraction of those forces, helping pave the way for more deterministic engineering of nanosuspension droplet behavior.
“Nobody had captured the pinning of an advancing contact line in an atomistic scale simulation and computed during that process the relevant forces. We’re really excited with our result.”
Webb’s simulations show that a precursor film continues to advance across the surface even when the droplet is pinned in place by the particles within it. However, the rate of precursor advance is significantly reduced by the pinning particles.
Simulations by Shi are also revealing how particle size affects the process, and how the affinity between the particle and the surface on which the droplet is deposited affects the spreading of the liquid.
“This enables us to advance fundamental thermodynamic theory,” Webb says.
Shi’s simulations show what happens to atoms along the contact line between a liquid droplet and solid surface as a function of time, which means that she can detect, among other features, specific atomic transport mechanisms associated with contact line advancement.
While computer models are key to revealing fundamental atomic features of droplet behavior, they can create false data if the programmer isn’t faithful to the minutiae of how atoms truly behave, Webb says.
“Without explaining how things behave at the atomic scale, you can’t fully understand how the process works at the macro level,” he says. “If you don’t fully understand it, you can’t accurately model it. If you can’t model it, you can’t engineer optimized applications.”
In inkjet printing, particles in the drop are left behind in an ordered array after the ink has been deposited on the surface and the water has evaporated.
“If the particles that you’ve deposited in this fashion have the right photonic or electronic properties, then you’ve possibly synthesized a very, very useful device,” Webb says.
Webb is studying a related phenomenon: What happens at the atomic level when a droplet hits a solid surface with speed, like in thermal sprayers used to apply ceramic or metal coatings to other materials? Fundamental questions about this phenomenon still exist.
“If you have a drop of a given size and material, how fast do you have to throw it to cause it to fracture on contact? If it’s slow enough, it might just spread out,” he says. “If a droplet is thrown too fast and splashes, it could be a disaster or it could be precisely what you desire – it depends on the application.” In such situations, one may also be concerned with damage generated in the underlying substrate. Webb is hoping that research into droplet impact will refine the ability to precisely control the final shape of deposited materials and enable advanced materials design.
Posted on:


