How DNA “bends” without breaking under UV radiation
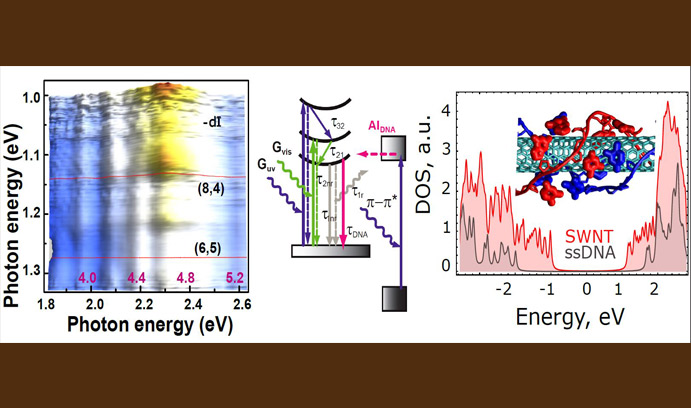
Two-color photoluminescence spectroscopy (left), combined with quantum mechanical calculations (center), explains the autoionization that enables self-assembled DNA complexes (wrapped around a single-wall carbon nanotube, at right) to recover after absorbing ultraviolet radiation.
DNA, which stores genetic information in the majority of organisms on Earth, is not easily destroyed. It readily absorbs ultraviolet (UV) radiation, but finds ways to recover.
To combat radiation’s damage, cells have developed DNA repair mechanisms, as well as mechanisms to remove the energy before it breaks down the DNA.
One of these mechanisms, autoionization, is the process by which the DNA macromolecule in an excited state spontaneously emits one of its electrons, releasing a huge amount of energy. Understanding this mechanism is critical to investigating and mitigating the effects of radiation on living organisms.
A team of researchers from Lehigh, the University of Central Florida, the National Institute of Standards and Technology (NIST) and the University of Rochester set out to understand the ability of DNA to remain a stable carrier of genetic information despite the potentially damaging role of UV radiation. They have reported their findings in a paper recently accepted for publication in Nano Research.
The paper, titled “Two-color spectroscopy of UV excited ssDNA complex with a single-wall nanotube photoluminescence probe: Fast relaxation by nucleobase autoionization mechanism,” was authored by Tetyana Ignatova, Slava V. Rotkin and Michael Blades of Lehigh; Alexander Balaeff of Central Florida; Ming Zheng of NIST; and Peter Stoeckl of Rochester. Ignatova received her Ph.D. from Lehigh in 2013 and is now a postdoctoral researcher at the University of California at Irvine. Rotkin is a professor of physics and also of materials science and engineering, while Blades is a Ph.D. candidate. Stoeckl took part in the National Science Foundation-supported Research Experiences for Undergraduates summer program at Lehigh.
The team studied self-assembled complexes of DNA wrapped around single-wall carbon nanotubes utilizing a special technique: two-color photoluminescence spectroscopy. Using UV and visible light simultaneously to probe the sample, they gained a perspective no one had previously observed in single-color experiments. The team later developed a quantum mechanical theory to support the experimental data and was able to confirm a very fast DNA autoionization rate.
“Being able to establish the efficiency of the autoionization process is a key step in understanding how UV-excited DNA can ‘cool down’ without breaking, thus keeping its normal biological functions,” said Rotkin.
Because of its potential for monitoring DNA excitation, autoionization and chemical damage, said Rotkin, the team’s innovative approach could prove important to researchers in the fields of medicine, evolutionary biology, and space exploration.
For biomedical purposes, the ability to study autoionization could help scientists determine survivable levels of UV radiation for different cell types as well as ways of mitigating the effects of that radiation.
From an evolutionary perspective, it is important to understand the dissipation mechanisms which were crucial during primordial cell evolution when UV radiation was orders of magnitude more intense than today and when DNA repair mechanisms were, scientists believe, non-existent.
And for space exploration, it is important to develop strategies for cellular and organismal safety in harsh radiation conditions.
It took Rotkin’s team three years to collect data and analyze the effects. “We were surprised to observe changes in the nanotube’s optical properties as the UV light was applied to the samples: it seemed like something was ‘stealing’ the emitted light under the second-color UV illumination,” said Rotkin.
He added: “This field is still extremely underexplored. No one had seen this before and we had to hypothesize about the two-color data for a while, putting forward and experimentally rejecting various models in order to find the right interpretation.”
It was only when they assumed that the DNA was the source of the changes to the nanotube’s optical properties—and rejected a widely accepted model—that the researchers were able to fully understand nanotube optical quenching.
DNA is very useful for studying nanotubes. A strand of DNA wrapped around a single carbon nanotube— a miniature cylindrical carbon structure that has a hexagonal graphite lattice and walls that are only one atom thick—will hold the nanotube in water and allow it to have practically the same good optical properties as pristine material.
“For years it has been commonly accepted that DNA is an ‘inert’ carrier for nanotubes and that DNA holds the nanotube in water without changing its properties,” added Rotkin. “It took several years for our team to part with this idea, because it was so broadly accepted. Finally, after a series of additional experiments, the data clearly indicated the origin of the modulation to be the DNA itself.”
On the heels of this discovery, the researchers have shifted their focus to see how two-color photoluminescence spectroscopy might be used to further probe the properties of DNA.
“It is now understood that different DNA nucleobases show different autoionization properties,” said Rotkin. “We anticipate this will create unprecedented non-invasive biomolecular tools for solving critical problems of biophysics of nucleic acids.”
The study was funded by the National Science Foundation (NSF:ECCS) within a project called “Fundamental physics and biosensing applications of composite fluorescent nanomaterials – rare-earths combined with DNA-enclosed carbon nanotubes.”
Story by Lori Friedman
Posted on:

